Short description
New kinetic approach for evaluation of hazard indicators based on merging DSC-ARC or large scale tests.
Two main steps of kinetic-based approach are:
(i) determination of kinetic parameters of the ecomposition reaction which allow quantifying rate of the heat production and (ii) the heat balance which allows quantifying the rate of the heat loss in g-, kg- and tonne-scales.
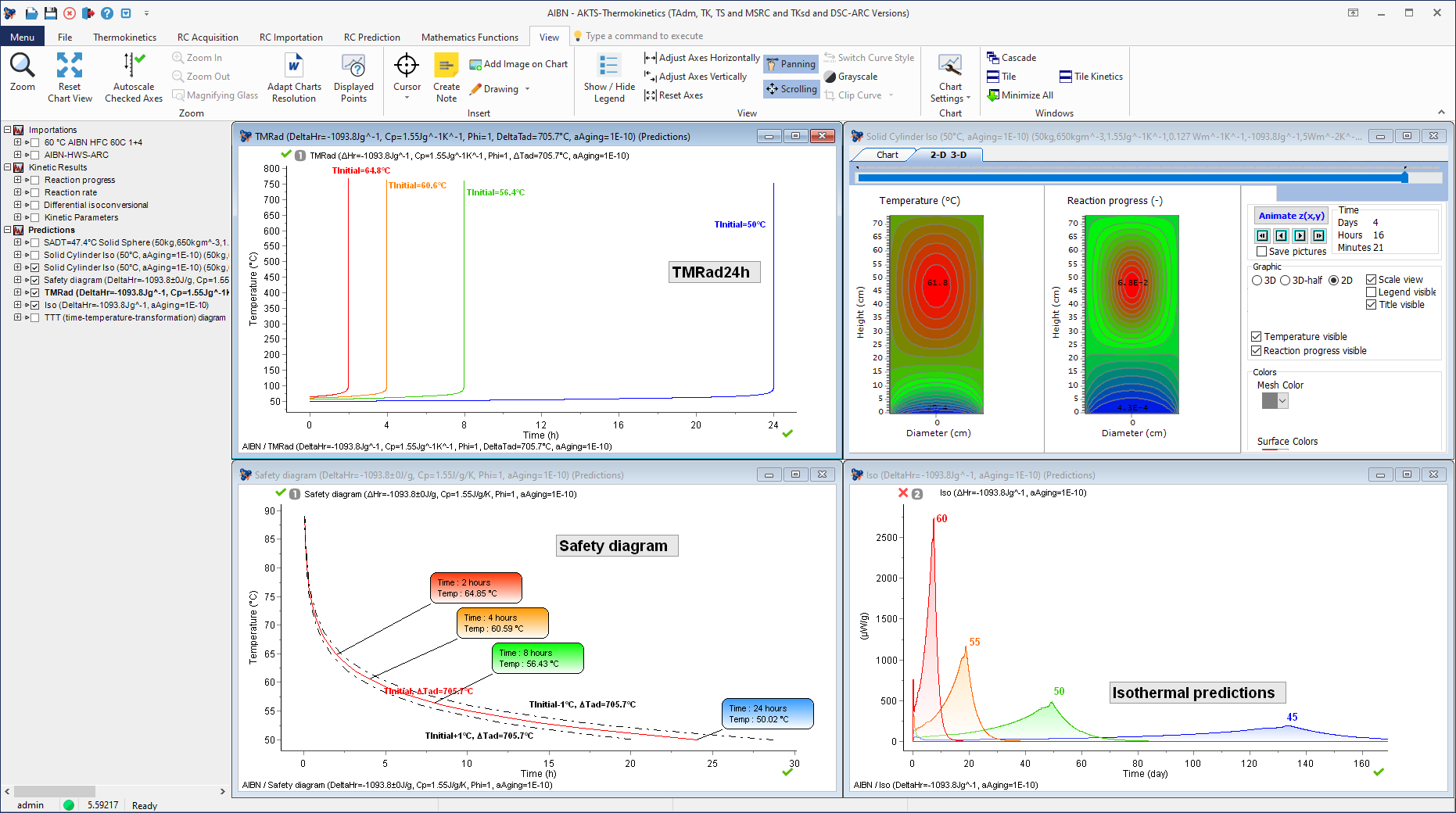
Time to Maximum Rate under adiabatic conditions (typically TMRad 24h, with Phi=1) from the results of the experiments performed in mg scale by e.g. Differential Scanning Calorimetry (DSC (or Calvet calorimeter, TAM, etc.)) and Accelerating Rate Calorimetry.
New method for determination of the Self-Accelerating Decomposition Temperature (SADT) of AIBN on the basis of Heat Flow Microcalorimetry and Accelerated Rate Calorimetry experiments.
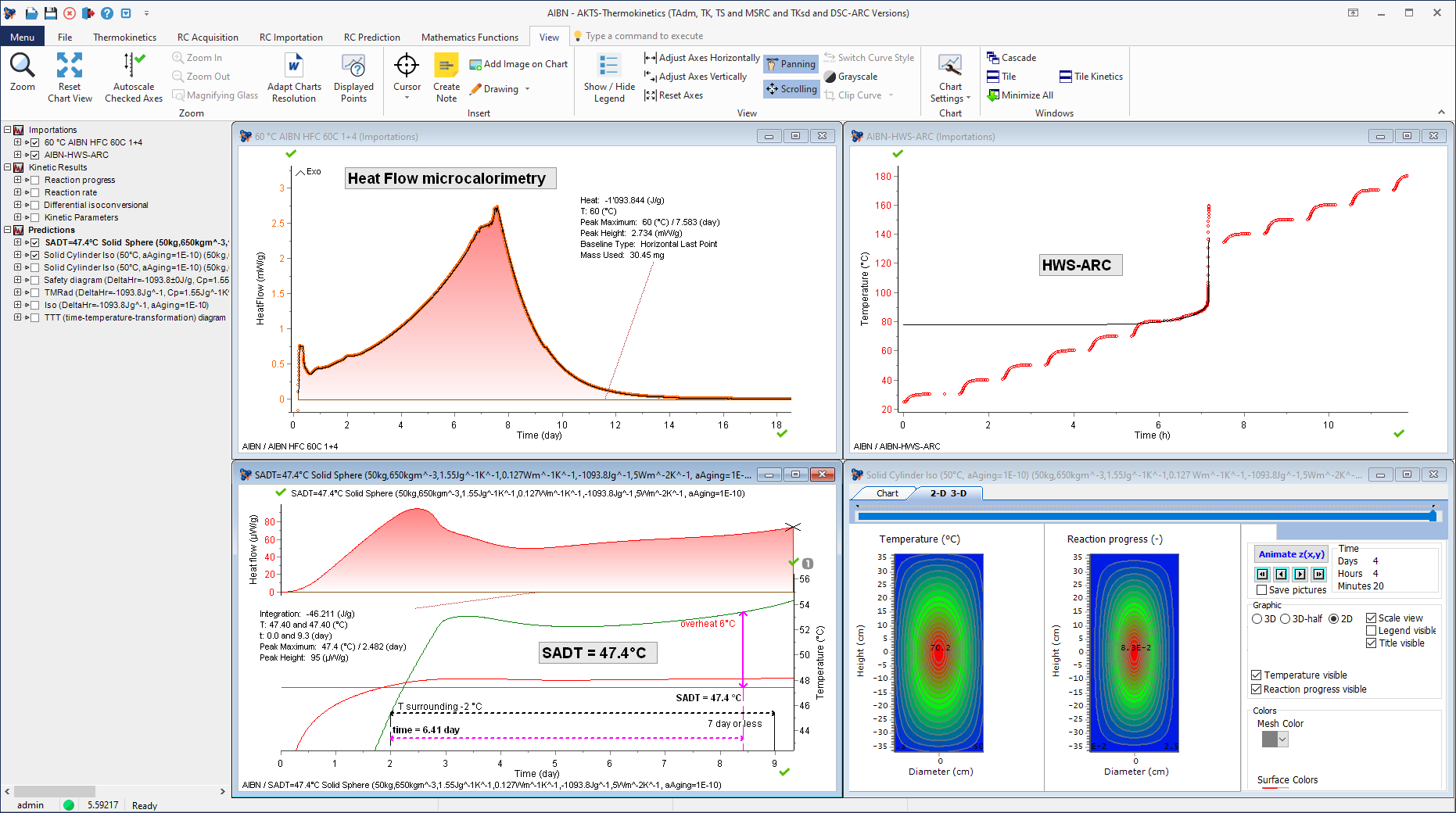
Time-To-Maximum Rate under adiabatic conditions (TMRad) as a function of initial AIBN temperature (including thermal simulations and isothermal predictions).
Previous studies:


References:
B. Roduit, D. Rickenbach, P. Folly, A. Sarbach, K. Kurko, R. Baltensperger, Application of data loggers, heat balance and kinetic analysis of HFC, DSC or ARC data for evaluation of safety parameters of energetic materials. Remote monitoring of the remaining shelf-life.
Click and download contribution to BAM project for more information about AIBN
References:
B. Roduit, M. Hartmann, P. Folly, A. Sarbach, P. Brodard, R. Baltensperger, Thermal Decomposition of AIBN, Part B: Simulation of SADT Value Based on DSC Results and Large Scale Tests according to Conventional and New Kinetic Merging Approach, Thermochim. Acta, 621 (2015) 6-24.
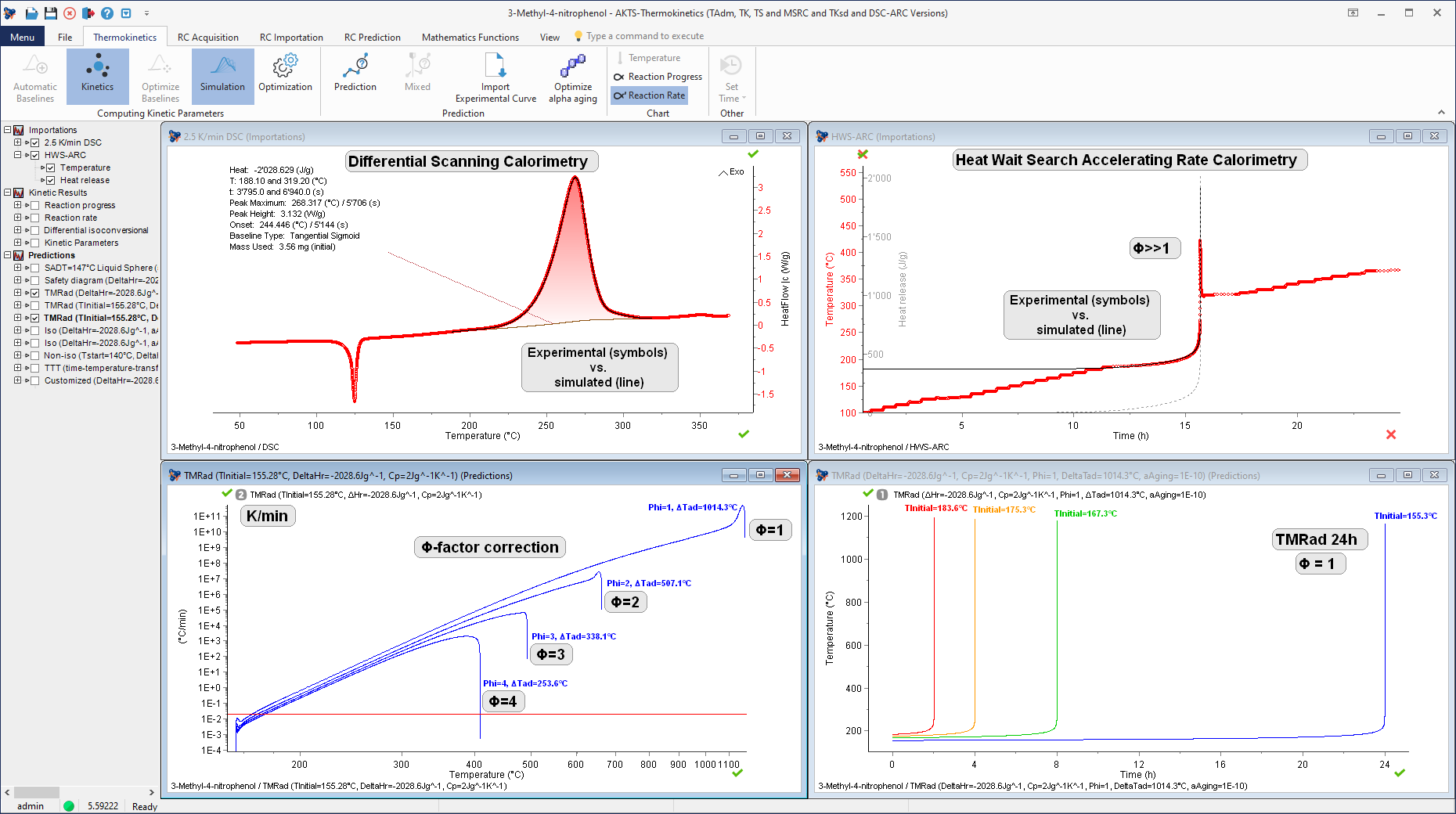
New method for determination of the Time-To-Maximum Rate under adiabatic conditions (TMRad) of 3-Methyl-4-nitrophenol on the basis of Differential Scanning Calorimetry and Accelerated Rate Calorimetry experiments.
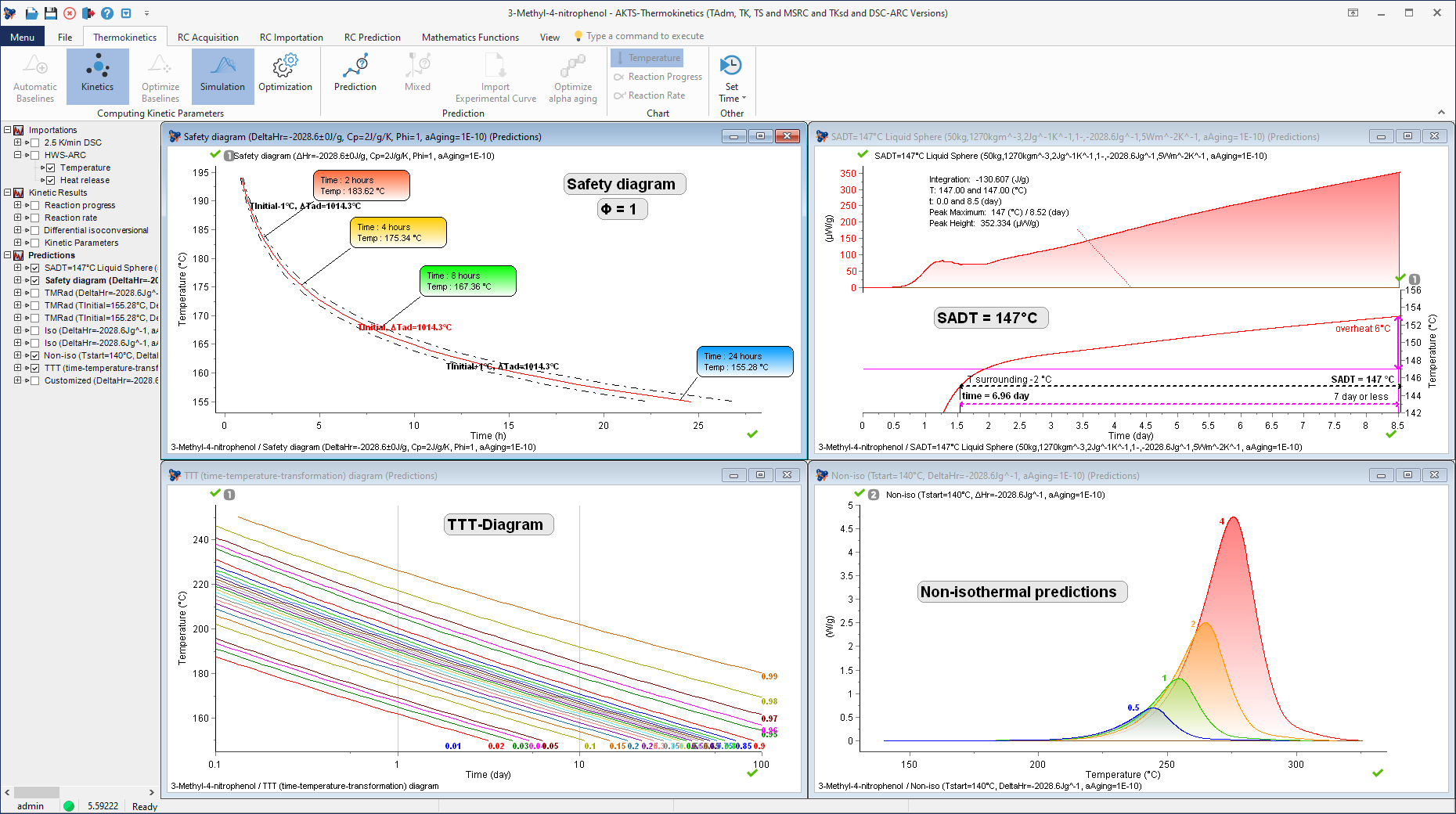
Determination of the Self-Accelerating Decomposition Temperature (SADT) of 3-Methyl-4-nitrophenol (including thermal safety diagram under adiabatic conditions, Time-Temperature-Transformation diagram and non-isothermal predictions).
Previous studies:


Click and download paper (*.pdf) for more information about 3-Methyl-4-nitrophenol.
References:
B. Roduit, F. Brogli, F. Mascarello, M. Schwaninger, T. Glarner, E. Irle, F. Tobler, J. Wiss, M. Luginbühl, C. Williams, P. Reuse, F. Stoessel, Estimation of Time to Maximum Rate under Adiabatic Conditions (TMRad) using Kinetic Parameters derived from DSC Investigation of Thermal Behavior of 3-Methyl-4-nitrophenol.


Click and download poster (*.pdf) for more information about 3-Methyl-4-nitrophenol.
References:
B. Roduit, F. Brogli, F. Mascarello, M. Schwaninger, T. Glarner, E. Irle, F. Tobler, J. Wiss, M. Luginbühl, C. Williams, P. Reuse, F. Stoessel, Estimation of Time to Maximum Rate under Adiabatic Conditions (TMRad) using Kinetic Parameters derived from DSC Investigation of Thermal Behavior of 3-Methyl-4-nitrophenol.
New method for determination of the Time-To-Maximum Rate under adiabatic conditions (TMRad) of Di-tert-butyl peroxide (DTBP/Toluene ) on the basis of Heat Flow Calorimetry (CALVET) and Accelerated Rate Calorimetry experiments (VSP).
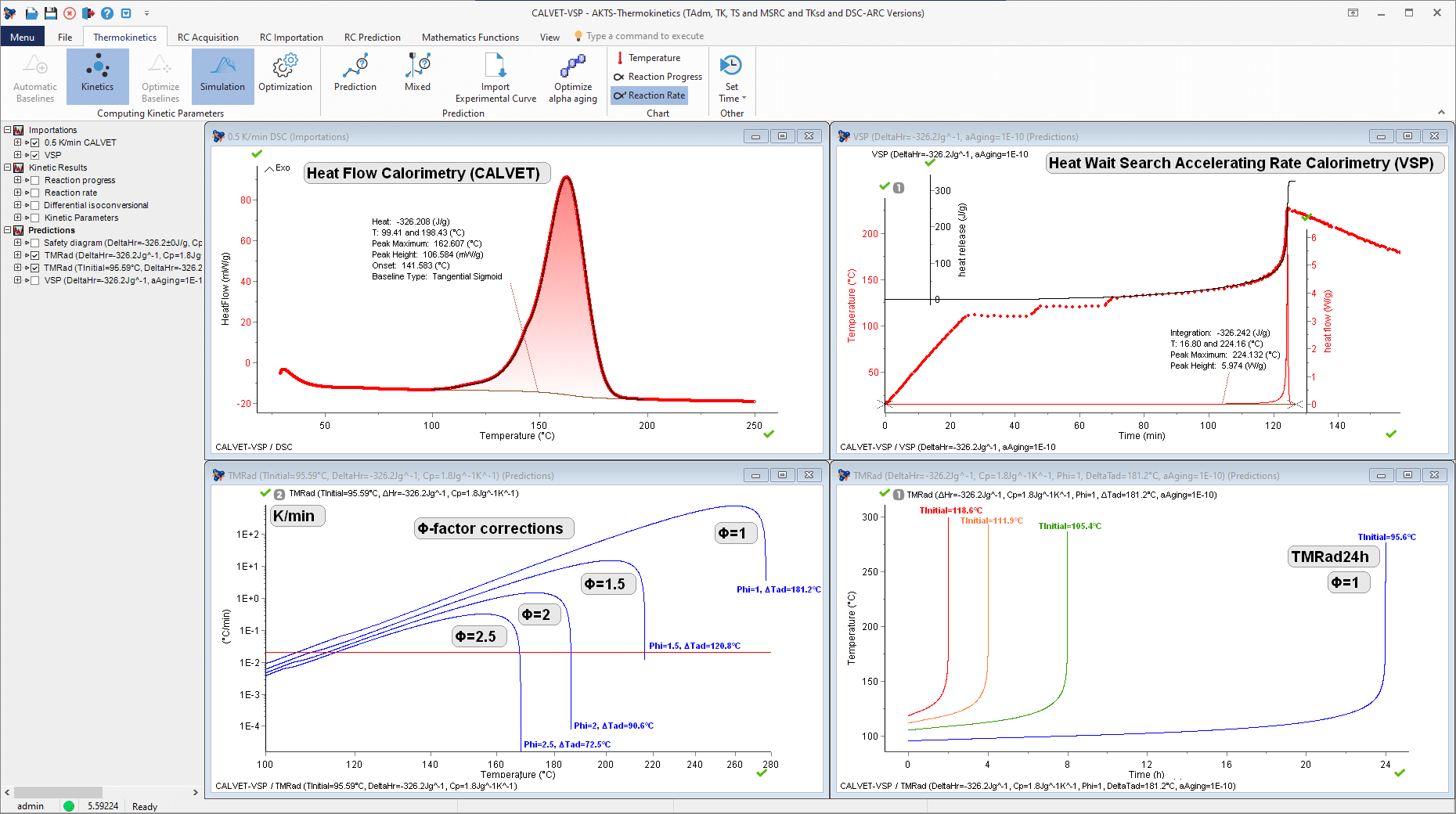
Determination of the Self-Accelerating Decomposition Temperature (SADT) of Di-tert-butyl peroxide (DTBP /Toluene) (including thermal safety diagram under adiabatic conditions, Time-Temperature-Transformation diagram and non-isothermal predictions).
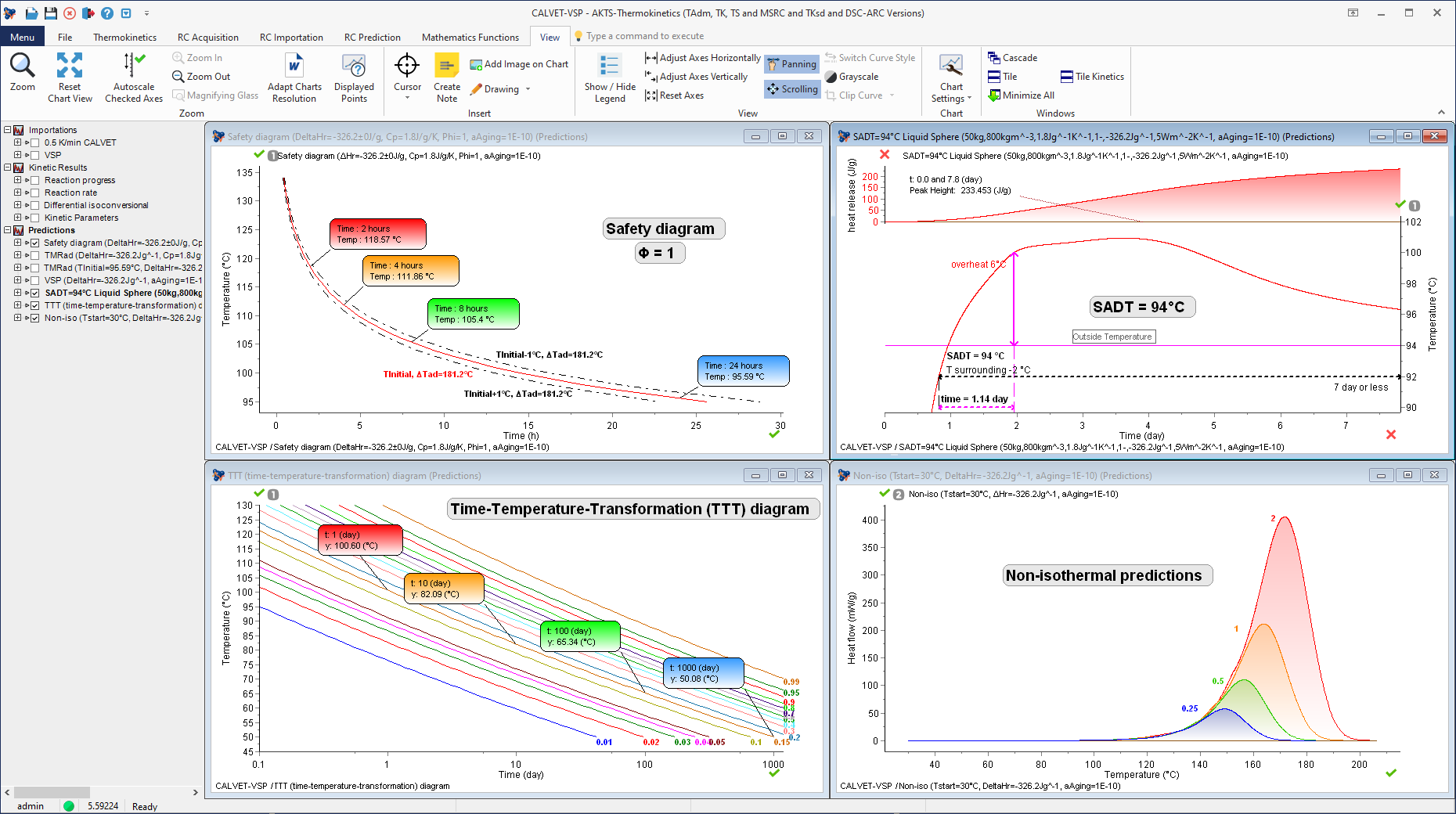
Previous studies:


B. Roduit, M. Hartmann, S. Kaneko, P. Folly, A. Sarbach, P. Brodard, S. Gomez, R. Marti, J.-N. Aebischer, Scale-up Based on Advanced Kinetics. Influence of DTBP/Toluene Ratio on the Thermal Behavior of Samples in mg, kg and ton-Scales.
References:
https://unece.org/fileadmin/DAM/trans/danger/publi/unrec/rev21/ST-SG-AC10-1r21e_Vol1_WEB.pdf
B. Roduit, Ch. Borgeat, B. Berger, P. Folly, B. Alonso, J.N. Aebischer, The prediction of thermal stability of self-reactive chemicals, from milligrams to tons, J. Therm. Anal. Calorim. 80 (2005) 91-102.
B. Roduit, Ch. Borgeat, B. Berger, P. Folly, B. Alonso, J. N. Aebsicher, F. Stoessel, Advanced kinetic tools for the evaluation of decomposition reactions, Determination of thermal stability of energetic materials, J. Therm. Anal. Calorim. 80 (2005) 229-236.
B. Roduit, Ch. Borgeat, B. Berger, P. Folly, H. Andres, U. Schädeli, B. Vogelsanger, Up-scaling of DSC data of high energetic materials, simulation of cook-off experiments, J. Therm. Anal. Calorim. 85, 1 (2006) 195-202.
B. Roduit, W. Dermaut, A. Lunghi, P. Folly, B. Berger, A. Sarbach, Advanced kinetics-based simulation of time to maximum rate under adiabatic conditions, J. Therm. Anal. Calorim., 93 (2008) 163-173.
B. Roduit, L. Xia, P. Folly, B. Berger, J. Mathieu, A. Sarbach, H. Andres, M. Ramin, B. Vogelsanger, D. Spitzer, H. Moulard, D. Dilhan, The simulation of the thermal behaviour of energetic materials based on DSC and HFC signals, J. Therm. Anal. Calorim., 93 (2008),143-152.
B. Roduit, P. Folly, B. Berger, J. Mathieu, A. Sarbach, H. Andres, M. Ramin, B. Vogelsanger, Evaluating SADT by advanced kinetics-based simulation approach, J. Therm. Anal. Calorim., 93 (2008) 153-161.
B. Roduit, P. Folly, A. Sarbach, B. Berger, F. Brogli, F. Mascarello, M. Schwaninger, T. Glarner, E. Irle, F. Tobler, J. Wiss, M. Luginbühl, C. Williams, P. Reuse, F. Stoessel, Estimation of time to maximum rate under adiabatic conditions (TMRad) using kinetic parameters derived from DSC-investigation of thermal behavior of 3-Methyl-4-Nitrophenol, Chem. Propel. Polym. Mat. 9, 1 (2011) 84-96.
B. Roduit, M. Hartmann, P. Folly, A. Sarbach, Parameters influencing the correct thermal safety evaluations of autocatalytic reactions, Chem. Eng. Trans. 31 (2013) 907-912.
B. Roduit, M. Hartmann, P. Folly, A. Sarbach, P. Brodard, R. Baltensperger, Determination of Thermal Hazard from DSC Measurements. Investigation of Self-Accelerating Decomposition Temperature (SADT) of AIBN, J. Therm. Anal. Calorim., 117 (2014) 1017-1026.
S. Vyazovkin, K. Chrissafis, M.L. Di Lorenzo, N. Koga, M. Pijolat, B. Roduit, N. Sbirrazzuoli, J.J. Suñol, ICTAC Kinetic Committee recommendations for collecting experimental thermal analysis data for kinetic computations, Thermochim. Acta, 590 (2014) 1-23.
B. Roduit, M. Hartmann, P. Folly, A. Sarbach, P. Brodard, R. Baltensperger, Thermal Decomposition of AIBN, Part B: Simulation of SADT Value Based on DSC Results and Large Scale Tests according to Conventional and New Kinetic Merging Approach, Thermochim. Acta, 621 (2015) 6-24.
B. Roduit, M. Hartmann, P. Folly, A. Sarbach, P. Brodard, R. Baltensperger, New kinetic approach for evaluation of hazard indicators based on merging DSC and ARC or large scale tests, Chem. Eng. Trans. 48 (2016).
B. Roduit, M. Hartmann, P. Folly, A. Sarbach, A. Dejeaifve, R. Dobson, K. Kurko, Kinetic analysis of solids of the quasi-autocatalytical decomposition type : SADT determination of low-temperature polymorph of AIBN, Thermochim. Acta, 665 (2018) 119-126.
F. Stoessel, Thermal Safety of Chemical Processes, Risk Assessment and Process Design, 2. Auflage – März 2020, WILEY-VCH Verlag GmbH & Co. CGaA.
